PDF) The HosA Histone Deacetylase Regulates Aflatoxin Biosynthesis Through Direct Regulation of Aflatoxin Cluster Genes

Sustainable, Stereoregular, and Optically Active Polyamides via Cationic Polymerization of ε‐Lactams Derived from the Terpene β‐Pinene - Winnacker - 2017 - Macromolecular Rapid Communications - Wiley Online Library

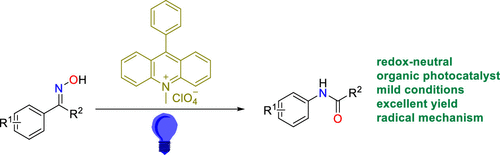
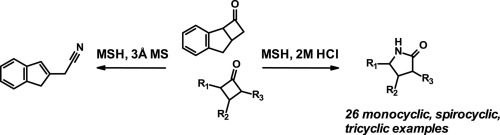
Cu(OTf)2-catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA). - Abstract - Europe PMC

Direct and Stereospecific Synthesis of N-H and N-Alkyl Aziridines from Unactivated Olefins Using Hydroxylamine-O-Sulfonic Acids. - Abstract - Europe PMC

Synthesis and reactivity of heterocyclic hydroxylamine-O-sulfonates in: Heterocyclic Communications Volume 20 Issue 3 (2014)

PDF) The HosA Histone Deacetylase Regulates Aflatoxin Biosynthesis Through Direct Regulation of Aflatoxin Cluster Genes