_zerfall-von-ammoniumchlorid-nh4cl-und-nachweis-von-nh3-und-hcl.jpg)
Zerfall von Ammoniumchlorid (NH4Cl) und Nachweis von NH3 und HCl from nh4cl Watch Video - HiFiMov.cc
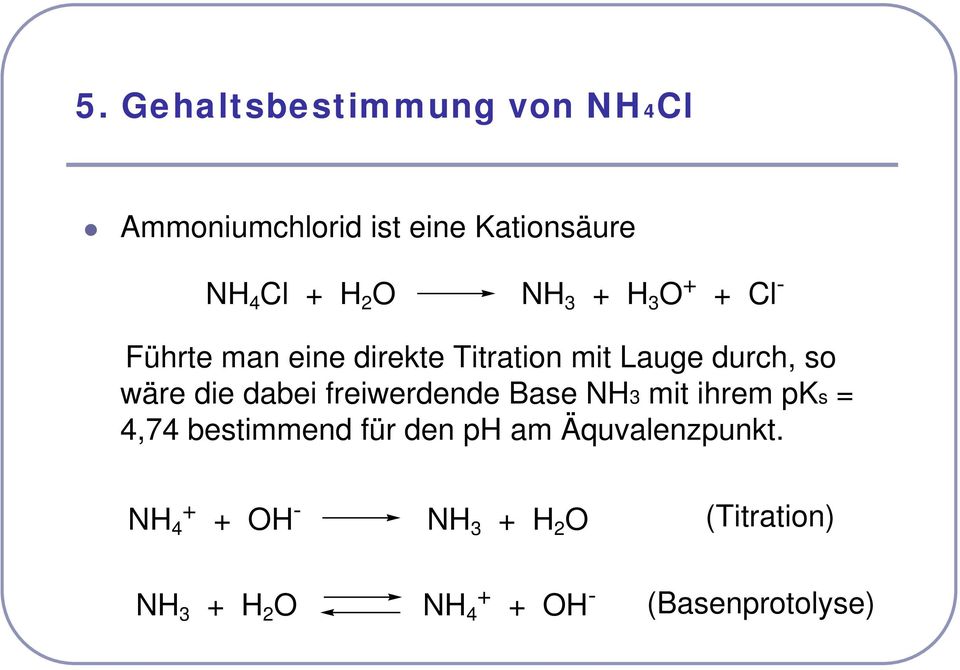
Säure-Base Titrationen. (Seminar zu den Übungen zur quantitativen Bestimmung von Arznei-, Hilfs- und Schadstoffen) - PDF Free Download

Pufferfish Rh glycoprotein-mediated uptake of the ammonia analog, [ 14... | Download Scientific Diagram
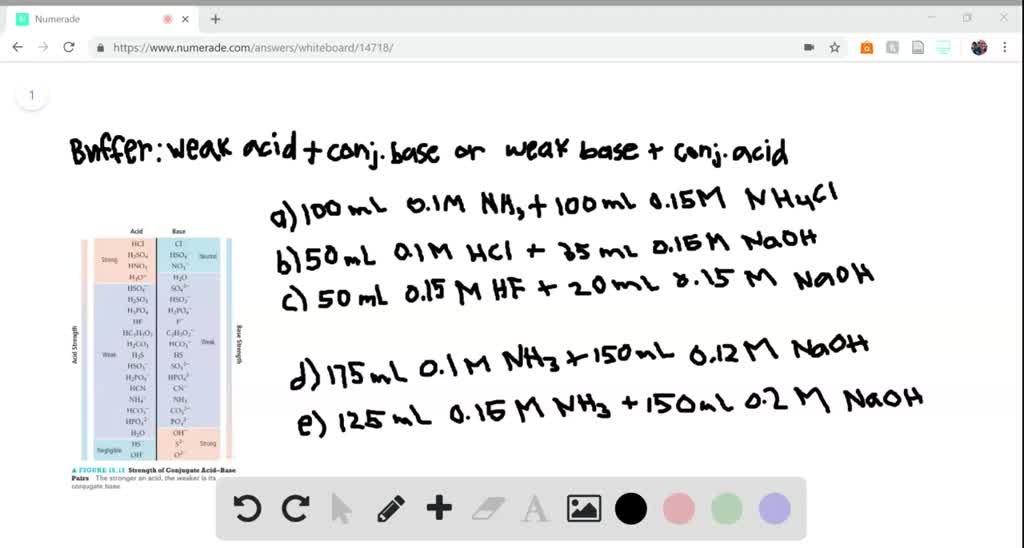
SOLVED:(i) You were asked to prepare 100.0 mL of a buffer solution with pH = 3.50. Which of the following system would give the best buffer? Explain your answer (A) CH3NH2 /
_nh4cl-naoh-et-nh3-hcl-1.jpg)
Zerfall von Ammoniumchlorid (NH4Cl) und Nachweis von NH3 und HCl from nh4cl Watch Video - HiFiMov.cc

Multiple alignment and hydropathy plot of pufferfish Rh glycoproteins.... | Download Scientific Diagram
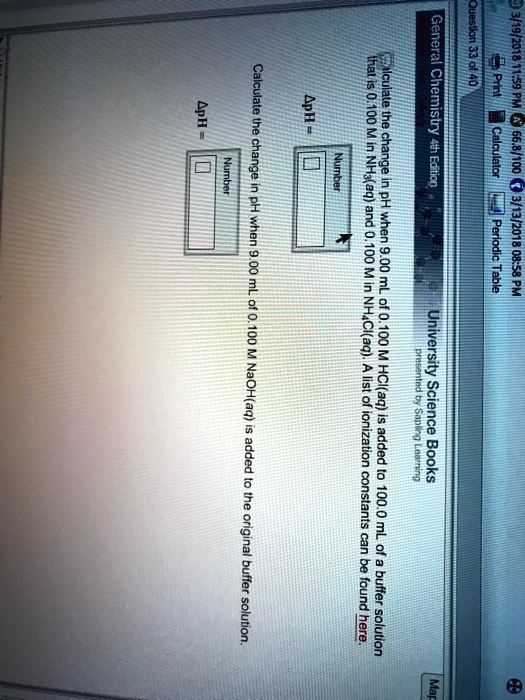
SOLVED:General 1 3/19/201 Calculale thaculad Chemistry Prnt pH the ApH = 0.100 M in 5 I I# Etticn Calaltnor in pH when and when Perlodic 3/13/2018 08-58 8 900 ML mL Table

SOLVED:Determine whether the mixing of each pair of solutions results in a buffer. a. 100.0 mL of 0.10 M NH3; 100.0 mL of 0.15 M NH4Cl b. 50.0 mL of 0.10 M

Solve the following systems of equations using elimination method. 0.5x + 0.8y = 0.44, 0.8x + 0.6y = 0.5