Effect of preferential exposure of anatase TiO2 {0 0 1} facets on the performance of Mn-Ce/TiO2 catalysts for low-temperature selective catalytic reduction of NOx with NH3 - ScienceDirect

A DNAzyme‐Based Colorimetric Paper Sensor for Helicobacter pylori - Ali - 2019 - Angewandte Chemie - Wiley Online Library
![Prodibio - Prodibio Start Up Nano [4 ampułki] - ogranicza wzrost niejonowego amoniaku NH3 i produkcję azotynów - sklep akwarystyczny Prodibio - Prodibio Start Up Nano [4 ampułki] - ogranicza wzrost niejonowego amoniaku NH3 i produkcję azotynów - sklep akwarystyczny](https://sklep.roslinyakwariowe.pl/zdjecia/prodibio_start_up_nano_4_ampulki_ogranicza_wzrost_niejonowego_amoniaku_nh3_i_produkcje_azotynow-i-36266-1.jpg)
Prodibio - Prodibio Start Up Nano [4 ampułki] - ogranicza wzrost niejonowego amoniaku NH3 i produkcję azotynów - sklep akwarystyczny
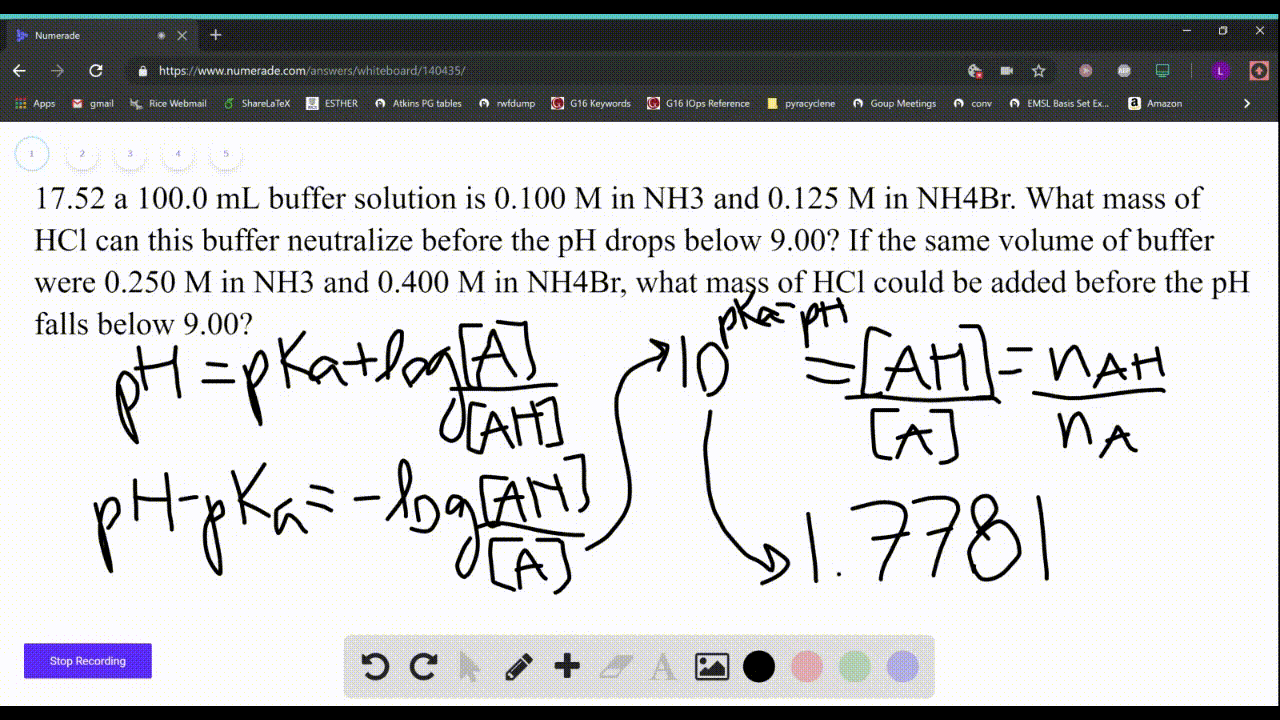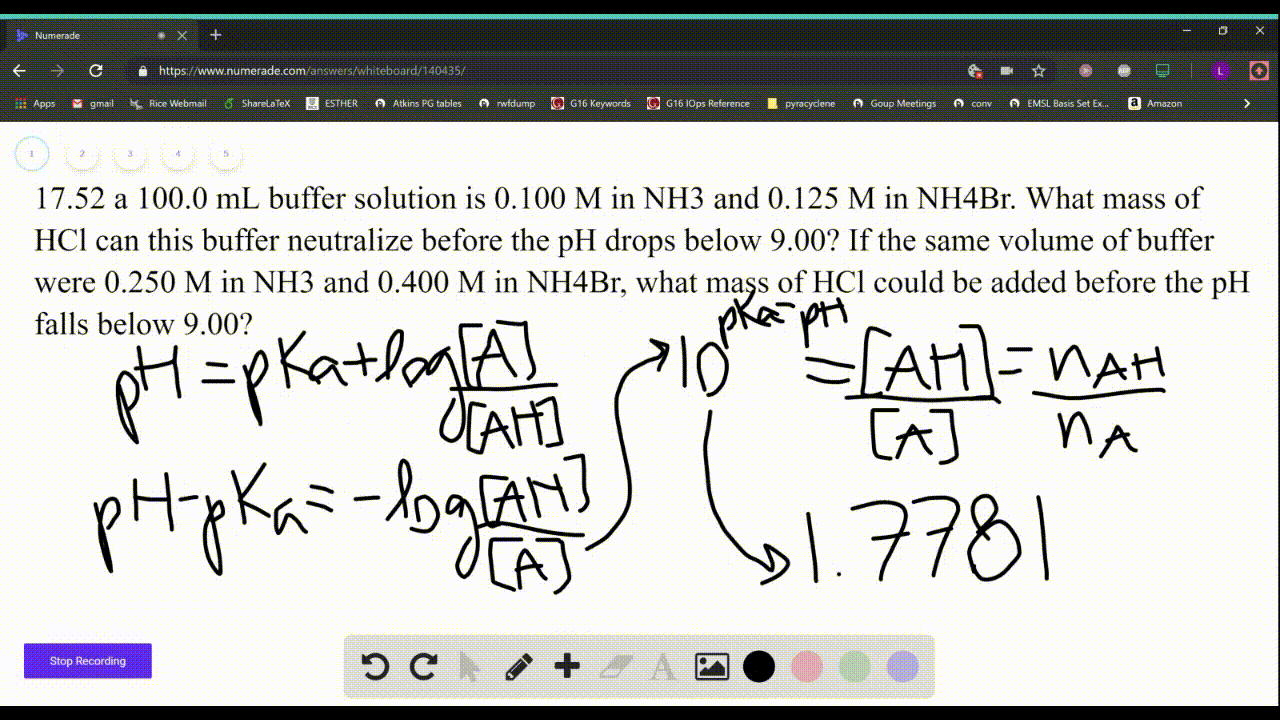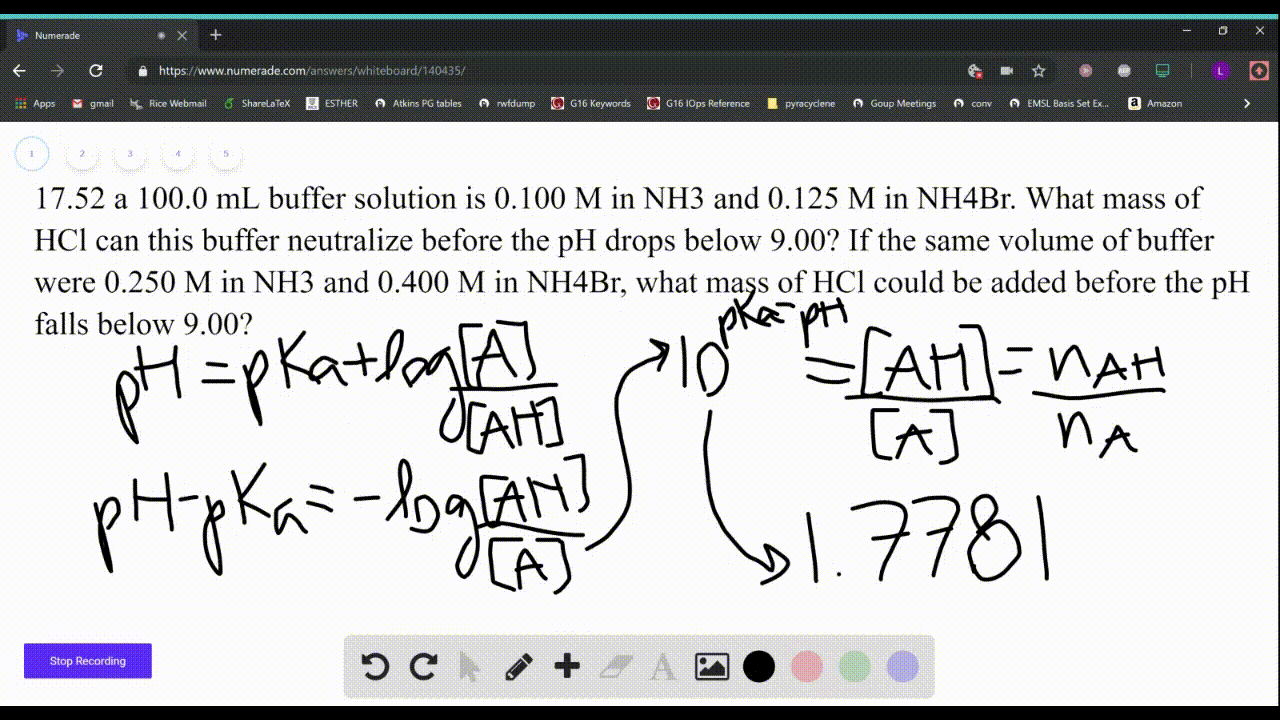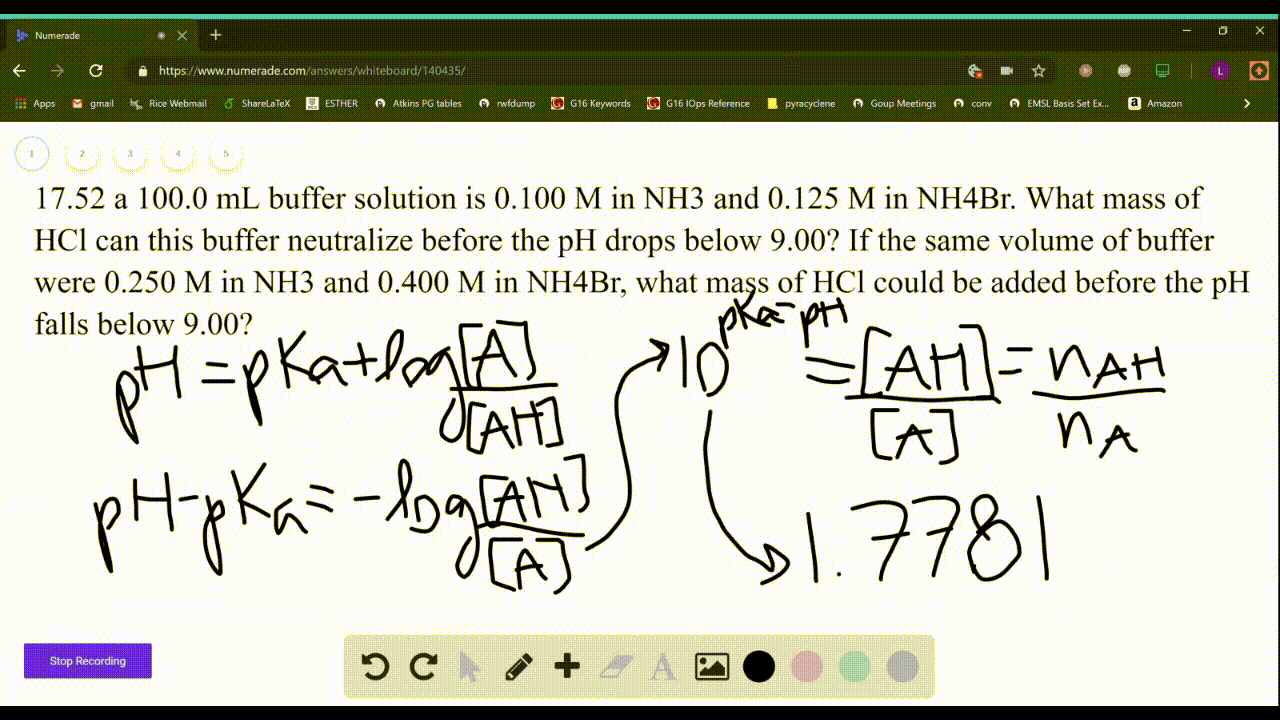
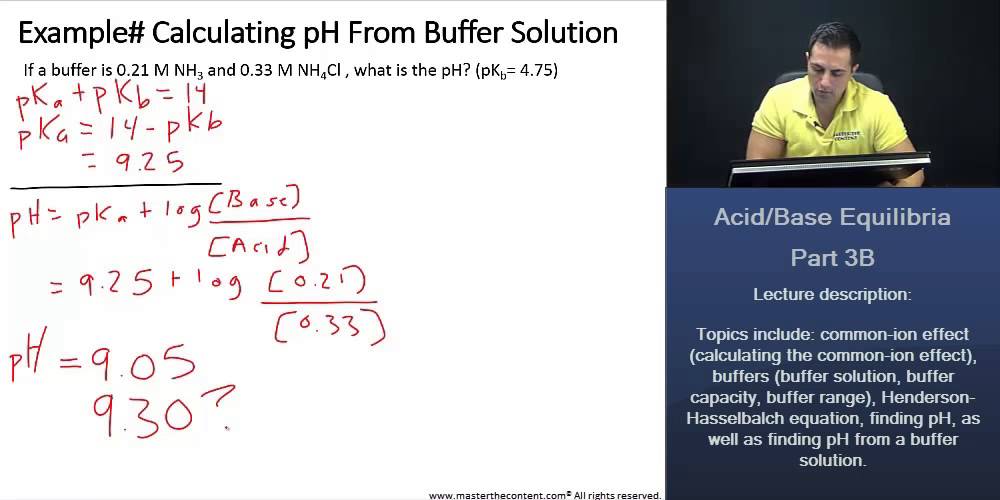
SOLVED: Which solution has the greatest buffering capacity?A) 0.335 M NH3 and 0.100 M NH4ClB) 0.085 M NH3 and 0.090 M NH4ClC) 0.540 M NH, and 0.550 M NH4ClD) 0.200 M NH,
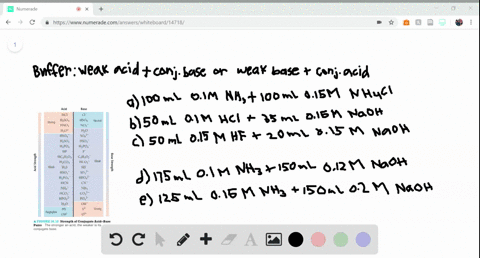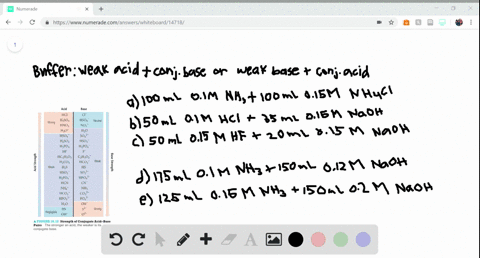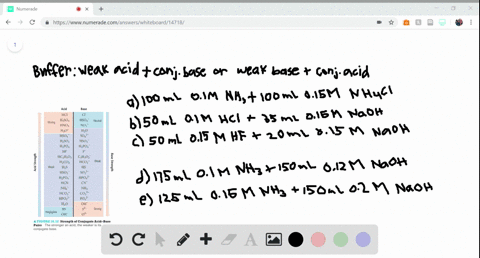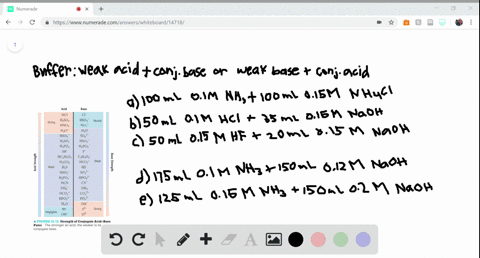
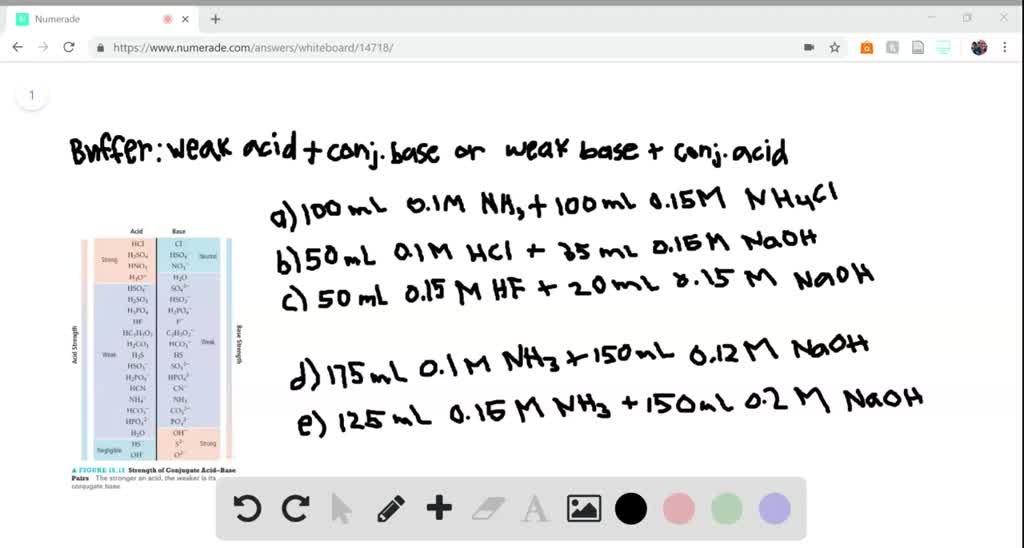
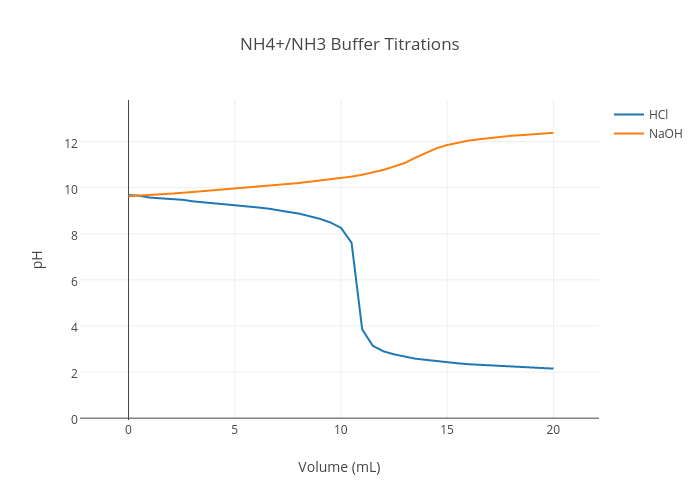
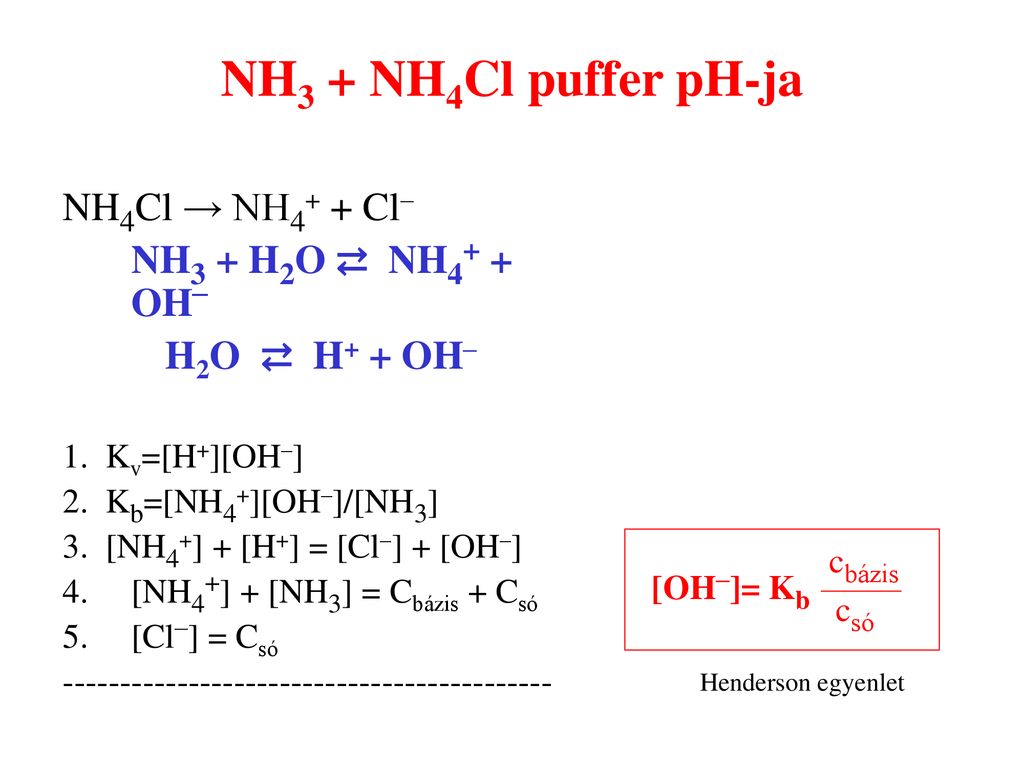
SOLVED:Determine whether the mixing of each pair of solutions results in a buffer. a. 100.0 mL of 0.10 M NH3; 100.0 mL of 0.15 M NH4Cl b. 50.0 mL of 0.10 M